
PRODUCTS
Soy lecithin liquid is a natural lecithin mixture e……

Soybean lecithin powder, also called soya lecithin ……

Product Name: Chitosan AzelateGrade: Cosmetic Grade……

Commodity Name: IscotrizinolChemical Name: Diethylh……

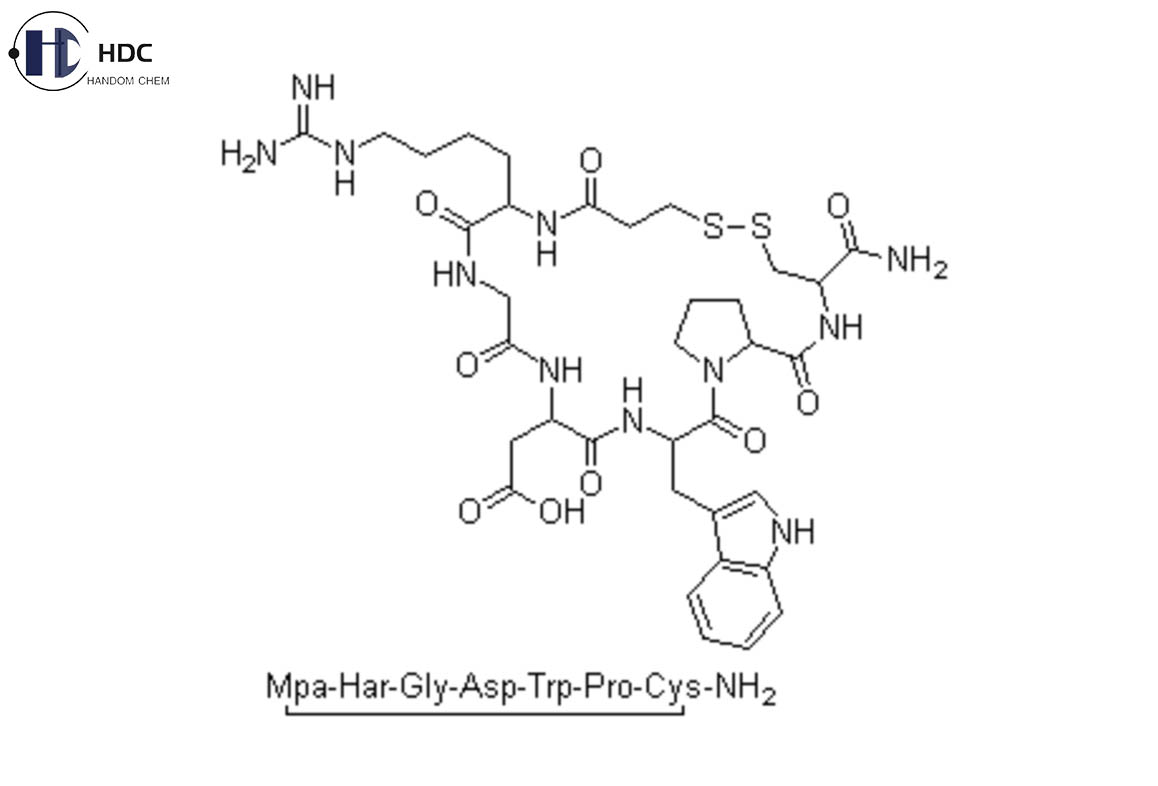
Product Name: Eptifibatide Acetate
CAS No.: 148031-34-9/157630-07-4/188627-80-7
EINECS No.: 641-366-7
Molecular Formula: C35H49N11O9S2
Molecular Weight: 831.962
Brief Introduction:
Eptifibatide is a synthetic cyclic heptapeptide, a glycoprotein IIb/IIIa receptor antagonist and a fibrinogen receptor antagonist with anti-platelet aggregation effects. Its main function is anti-platelet aggregation and can be used for anti-thrombotic treatment in acute coronary syndrome.
Specifications of our Eptifibatide Acetate:
Test Items
Specifications
Characteristic
This product is a white or off-white amorphous powder; odourless and hygroscopic. It is easily soluble in water and almost insoluble in chloroform or acetone.
Specific Rotation
Calculated based on anhydrous and acetic acid-free product, the specific rotation is between -52.0° and -56.0°
Identification
(1) This product has maximum absorption at wavelengths of 220nm and 280nm
(2) The retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference solution
(3) The infrared absorption spectrum of this product should be consistent with the spectrum of eptifibatide reference substance
(4) The mass spectrum molecular weight should be 832±1Da
Solution Clarity and Colour
The solution should be clear and colourless. If it is turbid, it should not be darker than the No. 2 turbidity standard solution; if it is coloured, it should not be darker than the yellow No. 1 or yellow-green No. 1 standard colorimetric solution.
Acidity
The pH value should be between 3.5 and 5.5
Moisture
Not more than 5.0%
Acetic Acid
Not more than 12.0%
Trifluoroacetic Acid
Not more than 0.5%
Residual Solvents
Methanol: Not more than 0.3%
Acetonitrile: Not more than 0.041%
N,N-Dimethylformamide: Not more than 0.088%
Amino Acids Ratio
Asparatic Acid (Asp): 0.8 ~ 1.2
Homoarginine (H-HomoArg-OH): 0.8 ~ 1.2
Glycine (Gly): 0.8 ~ 1.2
Proline (Pro): 0.8 ~ 1.2
Tryptophan (Trp): 0.8 ~ 1.2
Cysteine (Cys): 0.8 ~ 1.2
Related Substances
The peak area of impurity A and impurity H shall not be greater than 1.5 times (0.3%) of the main peak area of the control solution. The peak area of impurity B, impurity C, impurity D and impurity E multiplied by the correction factor (correction factor 1.23), impurity F (correction factor 0.86) and impurity G (correction factor 1.24) shall not be greater than the main peak area of the control solution (0.2%); The peak area of other single unknown impurities shall not be greater than the main peak area of the control solution (0.2%), and the sum of the peak areas of each impurity shall not be greater than 5 times the main peak area of the control solution (1.0%)
Polymer
The retention time of each component less than that of eptifibatide impurity G must not exceed 0.5%, and the total impurities should not exceed 1.0% (including impurity G)
Arsenic salt
Not more than 0.00015%
Bacterial endotoxins
The amount of endotoxin contained in every 1 mg of eptifibatide should be less than 0.2 EU
Microbial Limits
Total Aerobic Microbial Count: NMT 1000 CFU/g
Total Yeasts and Moulds Count: NMT 100 CFU/g
Gram-negative bacteria resistant to bile salts: Negative
Assay
Calculated as anhydrous and acetic acid-free, this product contains eptifibatide 98.0% ~ 102.0%
Indications:
This product is suitable for patients with coronary syndrome, regardless of whether they have acute coronary symptoms (unstable angina and myocardial infarction without Q wave), as well as those patients who have acute coronary symptoms and are taking drug treatment.
Packaging:
1g/bottle, 5g/bottle, 10g/bottle, 30g/bottle, 50g/bottle or 100g/bottle.
Storage Conditions:
Stored in unopened original containers in a cool dry place before using; kept away from direct sunlight, heat and moisture; preserved at 2℃ to 8℃ for short-term storage, -20℃±5℃ for long-term storage.
Shelf Life:
24 months if stored under above mentioned conditions.